Why ask for ASCOR® by name?
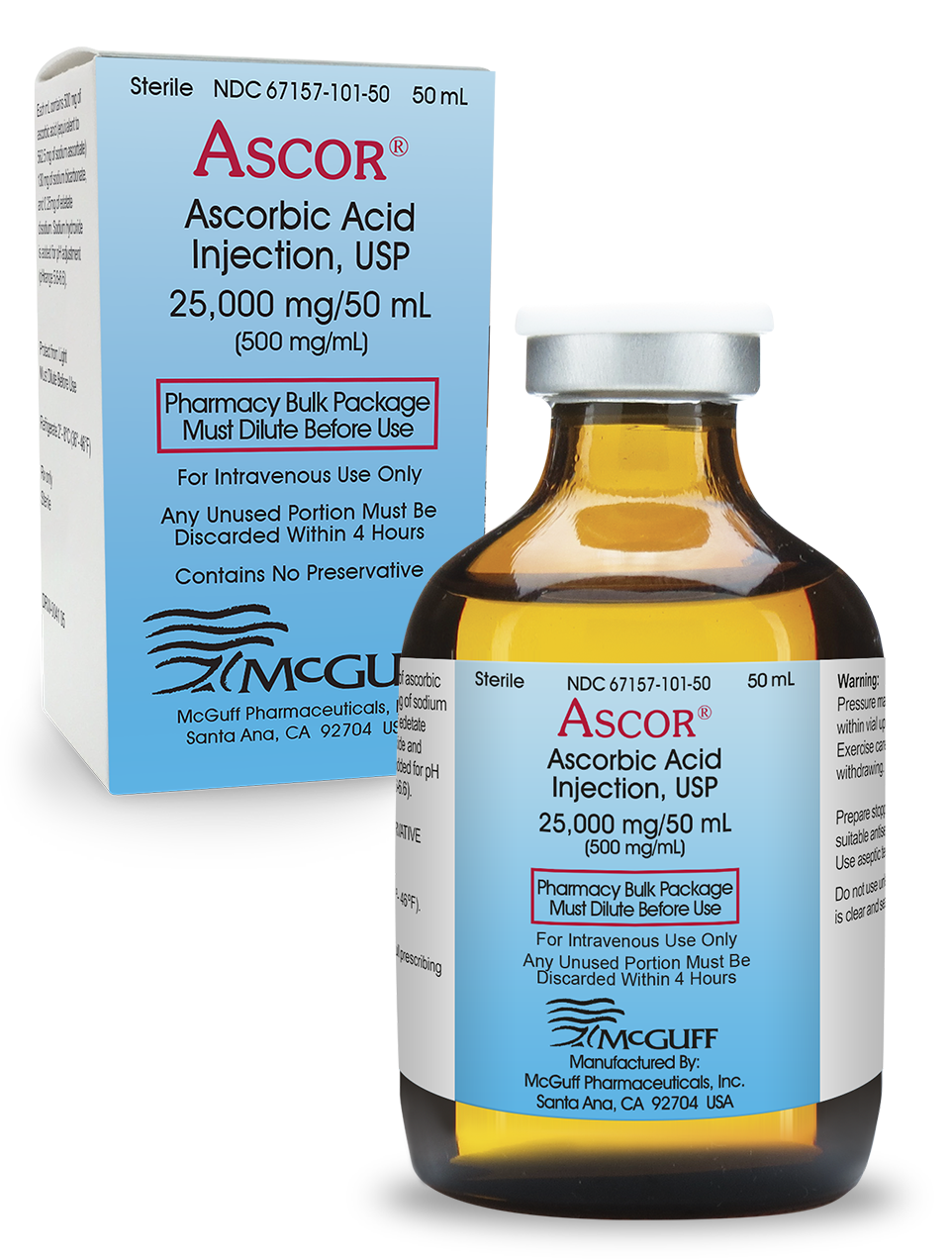
ASCOR® is the first and only FDA-approved ascorbic acid (Vitamin C) injection product. FDA approval means that the strength, safety, effectiveness and quality of ASCOR® are assured. ASCOR® is made in a manufacturing facility that is FDA-inspected and operates under federal current good manufacturing practices.
Other ascorbic acid (Vitamin C) injections are compounded drugs. Compounded ascorbic acid injection drugs have not undergone FDA pre-market review for safety, effectiveness, and quality. Additionally, the facilities where compounded drugs are made have not undergone an FDA pre-approval inspection to ensure the compounding facility is in compliance with FDA rules and regulations and meets all regulatory requirements for sterility, potency, pH and purity. 1
Because compounding pharmacies and 503b outsourcing facilities are subjected to a lower regulatory standard, compounded ascorbic acid injection products pose a greater risk to patients than the FDA-approved product ASCOR®. Compounding pharmacies and 503b outsourcing facilities that make and sell compounded ascorbic acid (Vitamin C) injections may also be in violation of state and federal laws that prohibit them from making an essential copy of an FDA-approved drug. 1
Ask for ASCOR® by name, the only FDA-approved ascorbic acid (Vitamin C) injection. There is an ample supply of ASCOR® and it can be easily ordered through authorized distributors or directly from McGuff Pharmaceuticals.
In addition to the peace of mind of using an FDA-approved drug, ASCOR® offers the following benefits:
- ASCOR®'s active pharmaceutical ingredient (raw material) and finished drug product have been tested using scientifically validated methods for the presence of amino acids, proteins, polypeptides, and corn allergens. None of these allergens were detected in ASCOR®
- ASCOR® does not contain dyes, fillers or preservatives
- ASCOR® is a non-GMO product
For your peace of mind, ask for ASCOR® by name.
The Importance of Vitamin C
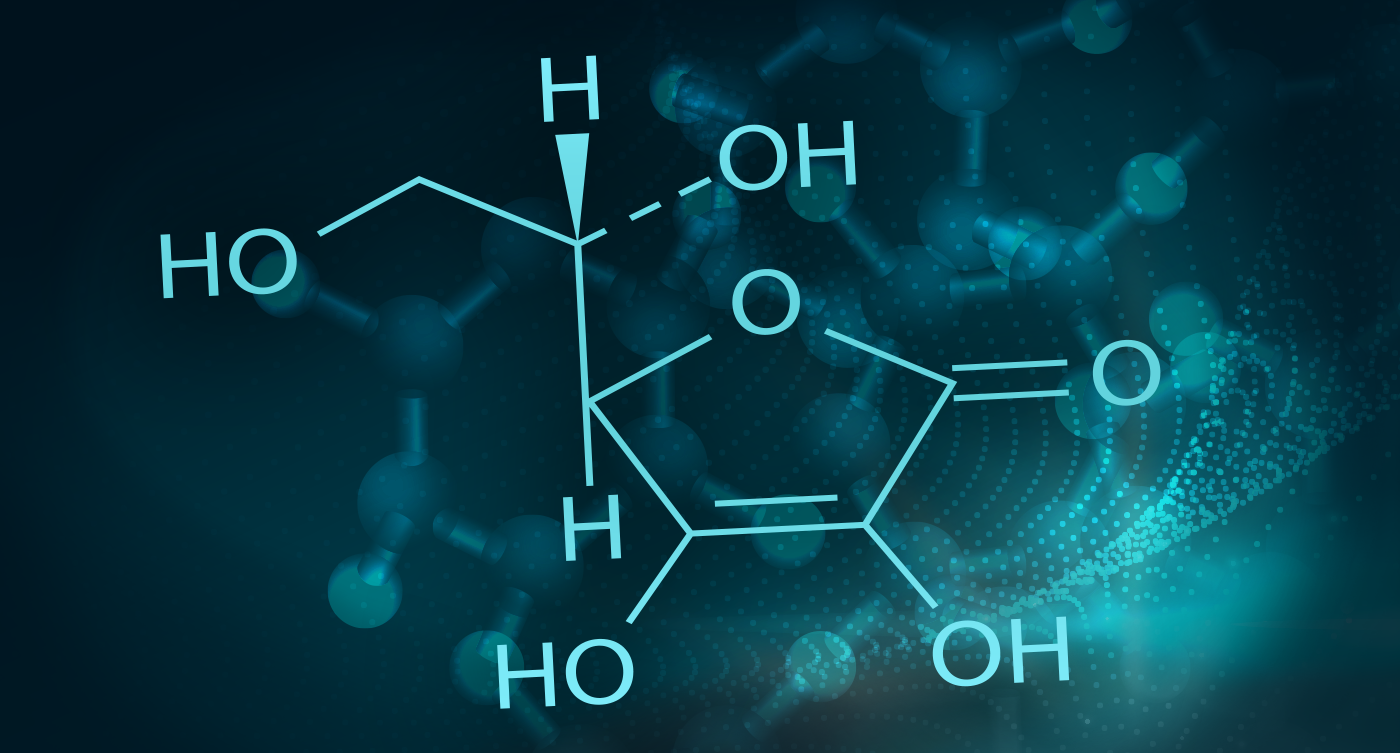
Vitamin C (L-ascorbic acid) is a water-soluble vitamin with several important physiological functions. Vitamin C is required for the biosynthesis of collagen, L-carnitine, and certain neurotransmitters. Vitamin C is also an important antioxidant and has been shown to regenerate other antioxidants within the body, including alpha-tocopherol (vitamin E). Additionally, vitamin C contributes to protein metabolism, improves the absorption of nonheme iron and plays an important role in immune function. 2 Click here to learn more about vitamin C.
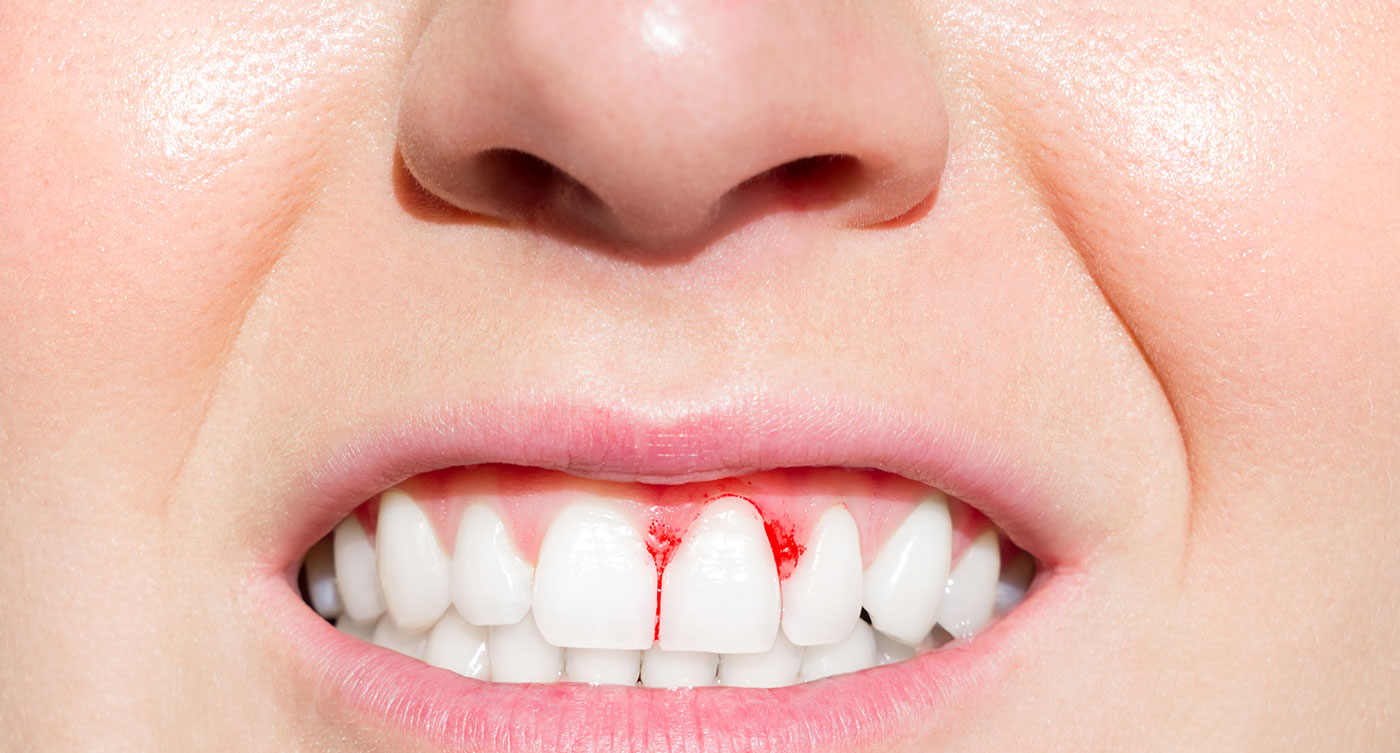
Insufficient vitamin C leads to scurvy. If left untreated, serious problems can develop such as anemia, widespread connective tissue weakness, capillary fragility, gum disease, and skin hemorrhages. 2, 3 Click here to learn more about scurvy and who is at risk for developing it.
- Compounded Drug Products That Are Essentially Copies of Approved Drug Products Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry (January 2018), https://www.fda.gov/regulatory-information/search-fda-guidance-documents/compounded-drug-products-are-essentially-copies-approved-drug-products-under-section-503b-federal, last accessed November 4, 2020.
- National Institutes of Health Vitamin C Fact Sheet for Health Professionals, https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/, last accessed September 30, 2020.
- National Institutes of Health, Genetic and Rare Diseases information Center, https://rarediseases.info.nih.gov/diseases/10406/scurvy, last accessed September 30, 2020.