There is a large variation in the bioavailability of oral and intravenous Vitamin C. This is because the transporting capability of the intestinal sodium ascorbate co-transporter, SVCT1, a surface glycoprotein that facilitates Vitamin C absorption, 1 is limited and achieves maximal saturation around oral doses of 500-1000 mg. 2 Intravenous administration of Vitamin C bypasses the limitations of SVCT1-induced bioavailability.
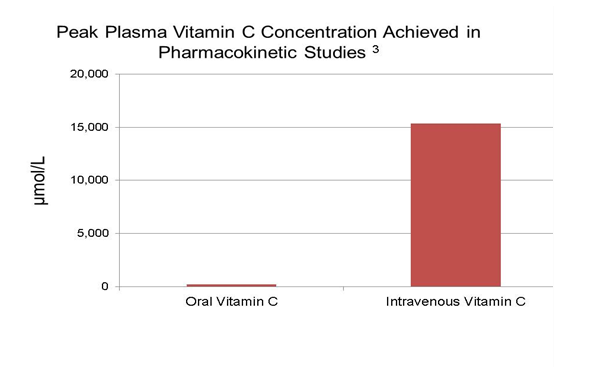
In a dose concentration and pharmacokinetic modeling study of healthy hospitalized volunteers, consumption of 5-9 servings of fruits and vegetables daily resulted in steady state Vitamin C plasma concentrations of 80 µmol/L or less and peak values did not exceed 220 µmol/L even after maximum oral administration of 3 grams, six times daily. In this same study, at a dose of 100 grams, intravenous administration of Vitamin C achieved peak plasma concentrations as high as 15,000 µmol/L, a plasma concentration 70 times higher than oral administration. 3
In another Vitamin C pharmacokinetics dosing study in healthy volunteers, the bioavailability of intravenous ascorbic acid was higher than oral ascorbic acid at single doses of 500 and 1250 mg but not at a dose of 200 mg. 2 Doses of ascorbic acid injection higher than 200 mg daily have not been approved by the US FDA.
- Savini, I., Rossi, A., Pierro, C. et al. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34, 347–355 (2008), https://doi.org/10.1007/s00726-007-0555-7.
- Levine, M., Conry-Cantilena, C., Wang, Y. et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 93:3704-3709 (1996), Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. (nih.gov).
- Padayatty, S., Sun, H., Wang, Y. et al. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann Intern Med 140: 533-537 (2004).